August 28, 2025 – Budapest Hungary
3DHISTECH Ltd., a pioneer in digital and computational pathology, is proud to announce that its Ki-67 Quantification Module has been granted IVDR certification for clinical use across the European Union. This recognition confirms the module’s safety, performance, and clinical relevance — giving pathologists confidence in a trusted digital tool designed to support cancer diagnostics and research.
Why Ki-67 Matters
Ki-67 is a protein found in actively dividing cells, making it one of the most important proliferation markers in pathology. Measuring Ki-67 can help answer crucial questions in oncology:
• How aggressive is a tumor?
• Will the patient benefit from more intensive therapy?
• How should treatment be monitored over time?
From breast cancer to neuroendocrine tumors, prostate cancer, gliomas, and lymphomas, Ki-67 continues to play a central role in guiding diagnosis, prognosis, and therapy decisions.
Certified to the Highest Standards
With IVDR approval, the Ki-67 module has passed the EU’s rigorous regulatory process, meeting strict requirements for:
• Performance and clinical validation
• Post-market surveillance
• Traceability and quality management
Importantly, the module itself does not make diagnostic, prognostic, or treatment recommendations. Instead, it provides pathologists with reproducible quantification and consistent labeling indices, supporting their expert judgment while ensuring that all final interpretations and clinical decisions remain in the hands of the pathologist.
A Module Designed with Pathologists in Mind
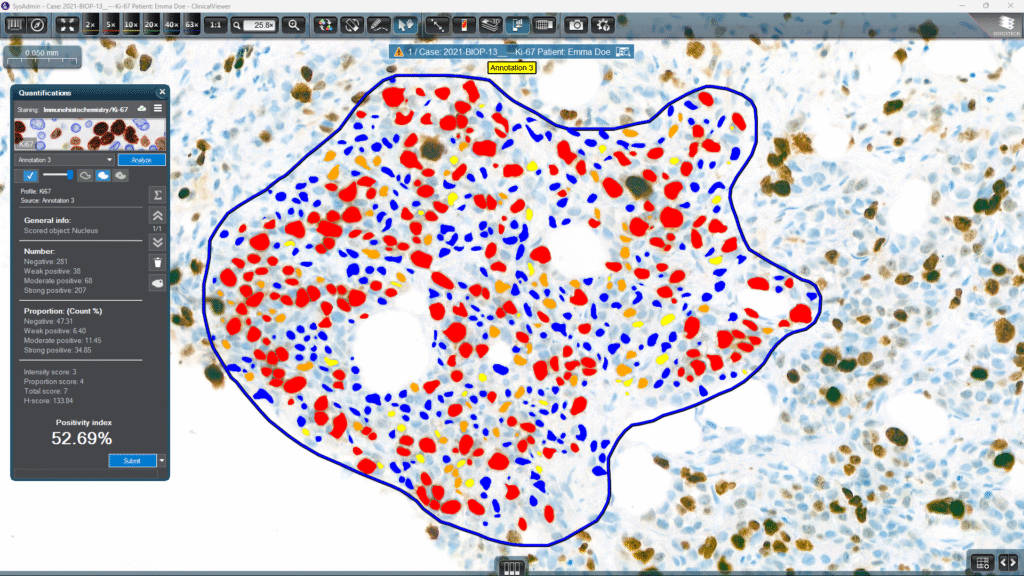
3DHISTECH’s IVDR-certified Ki-67 Quantification Module was built to integrate smoothly into the pathologist’s workflow. It allows for:
• Reproducible detection of nuclei and staining
• Consistent calculation of labeling index (percentage of positive tumor cells)
The result: faster, more consistent quantification, while maintaining the clinical oversight that pathologists demand.
(Regulatory disclaimer: Available in specific product variants and may not be available in every country 3DHISTECH is present, e.g. Canada.)
Tested, Trusted, and Validated
In a 2024 multicenter breast cancer study conducted on 100 cases, three board-certified pathologists evaluated Ki-67 both visually and with the 3DHISTECH algorithm, using three different PANNORAMIC DX diagnostic scanners and CaseManager DX software. The results demonstrated strong reproducibility across observers, platforms, and methods as reflected by the following correlation values and key performance metrics:
- Pathologist vs. pathologist: 0.77–0.91
- Confidence Interval (95% CI): 0.74–0.92
- Indicates excellent inter-observer agreement, showing that different pathologists reviewing the same cases produced highly consistent results.
- Scanner vs. scanner: 0.75–0.95
- Mean absolute difference: 1.2% labeling index
- Confirms hardware consistency, proving that results remain stable regardless of which PANNORAMIC DX scanner was used to digitize the slides.
- Algorithm vs. pathologist: 0.80–0.95
- Standard deviation: ±0.5% labeling index
- Demonstrates high concordance between the algorithm and human experts, supporting its reliability as a trusted computational assistant to the pathologist’s evaluation.
- Spearman’s correlation: 0.8767
- p-value < 0.001
- Reflects a strong statistical correlation, validating that ranking and scoring patterns from the algorithm closely match those of expert pathologists.
- Quadratic Weighted Kappa (QWK): 0.86604
- Interpretation: Near perfect agreement
- Indicates near-perfect agreement when weighing the degree of difference between scoring categories, a crucial metric in clinical reproducibility studies.
These results were reviewed and approved by the NNGYK (Nemzeti Népegészségügyi és Gyógyszerészeti Központ / National Centre for Public Health and Pharmacy) — Hungary’s national regulatory authority responsible for overseeing public health, pharmaceuticals, medical devices, and clinical diagnostics.
NNGYK’s approval is a vital regulatory milestone, as this organization plays a central role in ensuring the safety, performance, and compliance of healthcare technologies. Their evaluation confirmed that the Ki-67 Quantification Module meets strict European Union standards, providing the necessary foundation for IVDR certification.
This endorsement confirms that 3DHISTECH’s solution delivers accuracy, reproducibility, and reliability in real-world diagnostic workflows, supporting its deployment in clinical laboratories.
Beyond Ki-67: Building the Future of Computational Pathology
The Ki-67 module represents the first step in 3DHISTECH’s expanding vision for computational pathology. The company is exploring possibilities for future tools such as mitosis detection, lymph node metastasis recognition, and digital quantification of tumor-infiltrating lymphocytes. Once developed and appropriately validated, these additions are expected to extend the range of data-driven insights available to pathologists, further supporting precision oncology and improved patient care.
As highlighted in recent research (Díaz-Pérez JA, Kovács I, Tőkés T, et al. Virchows Archiv. 2021; 478:123–136. DOI: 10.1007/s00428-021-03213-3), computational tools such as Ki-67 quantification are paving the way for more reproducible, predictive, and prognostic pathology.
About 3DHISTECH
3DHISTECH Ltd. is a global innovator in digital and computational pathology. From high-performance scanners to advanced image analysis modules, 3DHISTECH develops solutions that help laboratories, hospitals, and research centers worldwide bring pathology into the digital age.
Press Release 3DHISTECH’s Ki-67 Quantification Module Receives IVDR Certification (PDF)
